🌍 WHO Updates 20th ICDRA Schedule
The World Health Organization (WHO) has announced an update regarding the 20th International Conference of Drug Regulatory Authorities (ICDRA). Originally scheduled for April 2026 in Riyadh, the event has been rescheduled and is now expected to be convened in 2027.
2027
New Target Year
20th
Edition
WHO
Convener
💊 Understanding ICDRA
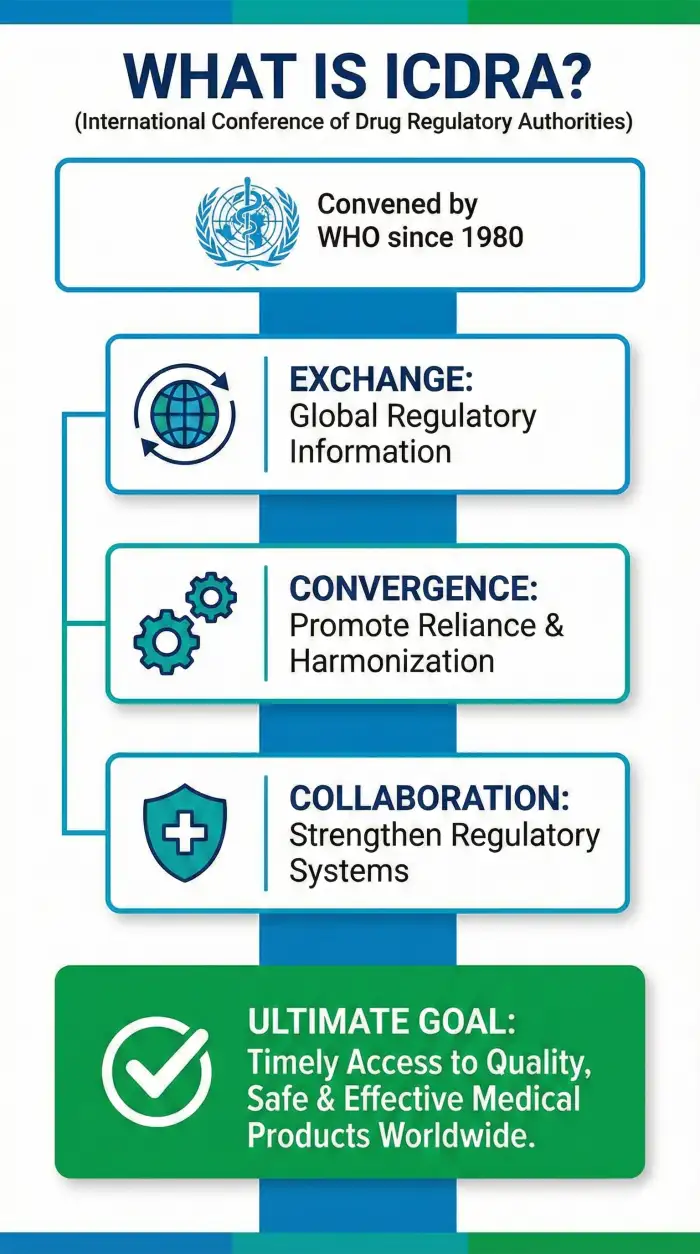
Since 1980, ICDRA has been a cornerstone for global health regulation, bringing together national and regional authorities to harmonize standards.
Global Platform
Connects regulatory authorities from WHO Member States to exchange information and discuss collaborative solutions.
Regulatory Convergence
Promotes reliance and harmonization of regulations to ensure medical products are safe, effective, and quality-assured.
Future Focus
WHO will issue a new call for expressions of interest to host the 20th edition, aiming to address emerging regulatory challenges.
📚 UPSC Corner: Prelims Quiz
1. The International Conference of Drug Regulatory Authorities (ICDRA) is co-convened by which organization?
Answer: B
2. What is the primary objective of ICDRA?
Answer: B
3. When was the ICDRA first convened?
Answer: B
4. The 20th ICDRA was originally scheduled to be held in which country before the update?
Answer: C
5. According to the latest update, when is the 20th ICDRA now expected to be convened?
Answer: B
📝 Mains Practice Questions & Model Answers
Q1. "Regulatory convergence in the pharmaceutical sector is essential for ensuring global health security." Discuss the role of international platforms like ICDRA in achieving this goal. (GS-2: International Relations/Health) - 250 Words
Model Answer Synopsis
Introduction
In a globalized world, diseases and medical products cross borders rapidly. Divergent regulatory standards can delay access to life-saving medicines. Platforms like ICDRA, convened by WHO, serve as a vital bridge.
Role of ICDRA
1. Harmonization: It encourages countries to align their safety and quality standards, reducing the duplication of clinical trials and approval processes.
2. Information Exchange: Regulators share data on adverse drug reactions and substandard medicines, acting as an early warning system.
3. Capacity Building: It supports developing nations in strengthening their regulatory frameworks through peer learning and expert guidance.
2. Information Exchange: Regulators share data on adverse drug reactions and substandard medicines, acting as an early warning system.
3. Capacity Building: It supports developing nations in strengthening their regulatory frameworks through peer learning and expert guidance.
Conclusion
By fostering "Regulatory Reliance"—where one regulator trusts the assessment of another—ICDRA accelerates access to affordable medical products, crucial for Universal Health Coverage (UHC).
Q2. What are the challenges faced by drug regulatory authorities in developing nations? How can international cooperation mitigate these challenges? (GS-2: Health) - 150 Words
Model Answer Synopsis
Challenges
Developing nations often face a lack of technical expertise, insufficient infrastructure for drug testing, and porous borders that allow the entry of falsified medicines.
Role of Cooperation
International cooperation (via WHO/ICDRA) allows for resource sharing. Wealthier nations can assist with technical training and technology transfer. Collective mechanisms help in creating unified standards, making it harder for low-quality drugs to penetrate the market.
Source Information: WHO Update (Feb 05, 2026)
World Health Organization
World Health Organization